Mouse brain aging transcriptional changes are fastest in the white matter
Here’s a nice article from Hahn et al., 2022, “A spatiotemporal map of the aging mouse brain reveals white matter tracts as vulnerable foci”, from Tony Wyss-Coray’s lab. They measured gene expression across 15 brain regions in mice at different ages. Here is a figure of their protocol:
Interestingly, they found that perfusion with the washout solution PBS to remove blood immediately after death, before brain removal and snap freezing by submersion in liquid nitrogen-cooled isopentane, led to substantially higher RNA quality.
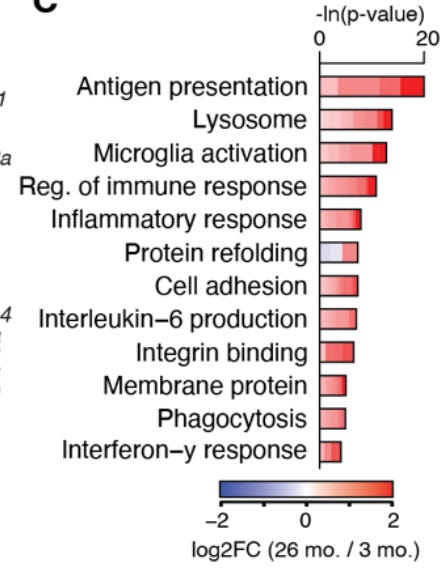
They did a bunch of bioinformatic analyses and eventually identified 82 genes that had strongly changed gene expression in aging across 10 or more brain regions. Here are the biological functions those 82 genes are the most associated with:
They called the change in the expression of these genes a “common aging score”. Here is a ranking of how rapidly transcriptional changes in these 82 genes occurred in different brain regions across aging:
As you can see, the corpus callosum (which is the largest white matter structure in the brain), and generally other white matter-heavy regions such as the caudate putamen, tended to have higher transcriptional aging severity scores than the other brain regions tested. As the authors note in their discussion:
Our data reveal that certain brain regions are selectively vulnerable to aging, with the white matter fiber tracts exhibiting a particular sensitivity. These areas are dense with myelinated axons and myelinating cells, forming the basis of neurotransmission across brain regions (Liu et al., 2017). The strong activation of immune- and inflammation-related genes, as well as differential expression of remyelination regulators like Gpr17 (Dziedzic et al., 2020; Rivera et al., 2021), suggest that the homeostasis of this region is compromised at old age. This could perturb myelin sheath integrity and potentially impair axonal signal transmission between regions as an early event in brain aging. In line with this hypothesis, rejuvenation of hippocampal oligodendrogenesis in aged mice via injection of young cerebrospinal fluid (CSF) improves long-term memory consolidation, thus demonstrating a causal role of compromised myelin on cognition (Iram et al., 2022).
I agree with the authors’ suggestion that there should probably be more focus on white matter when it comes to aging. A lot of evidence suggests white matter ages faster than grey matter, it seems very difficult to outright replace (because how would you replace the axon connections?), and it is potentially amenable to intervention.